Technology Transfer:
1. Optogenetics APP
Light delivery to deep brain areas is becoming increasingly more important, as more and more tools to optically manipulate neural activity become available. In particular, the currently evolving field of optogenetics provides very powerful and elegant ways to control neural activity with light. However, to take advantage of these tools in-vivo, correct and controlled light delivery to deep brain nuclei must be accomplished. Optogenetics is a tool which aids an investigator in estimating the required optical power for a given in-vivo experiment involving optogenetics or any other experimental approach that includes light delivery to deep brain areas via optical fibers. Different brain areas have different optical properties, which determine how light scatters and distributes in the brain tissue once it exits the optical fiber. To estimate the amount of light required for a given experimental design, knowledge about the specific scattering properties of the brain region, the wavelength of the light, the specific opsin to be used, as well as the properties of the optical fiber are required.
We created a computer program that computes the required optical power for a given in-vivo experiment based on these various parameters and suggests an optical power value for a particular experimental situation. The program models the maximal depth at which effective excitation of the opsin can be expected. Currently, the program is available as a Mac iOS APP which can be run on iPhones, iPads, and certain iPods. Versions for other operating systems are in progress.
APP Website:
www.optogeneticsapp.com
The APP is distributed by PopNeuron LLC, under license from the University of Colorado.
www.popneuron.com
2. Slice Chamber
Experiments involving patch clamp recordings from brain slices are typically done in a recording chamber that holds the brain slice in the optical path of a microscope, superfuses it with artificial cerebrospinal fluid (ACSF), and allows for the imaging of the tissue in which recordings are being performed. Most existing recording chambers continuously add fresh or reconditioned ACSF through one port of the chamber, while removing an equivalent amount of spent ACSF through a second port. Whenever expensive chemicals must be added to the bath solution such as pharmacological agents or caged compounds, experimenters reduce the total volume of ACSF required for the experiment as much as possible. We developed a novel slice recording chamber that reduces the amount of ACSF required for proper function down to 1.5 to 2.5 ml total. This reduction is accomplished by eliminating all tubing and holding containers, and instead oxygenating the ACSF directly in the recording chamber. Collaborators: Anna Dondzillo and Tim C. Lei
More information can be found at:
www.slicechamber.org
Patent:
A. Dondzillo, A. Klug, and T. Lei:
System and Methods for Conducting In-Vitro Experiments.
Awarded 10-4-2016. U.S. Patent Number 9,458,420. PDF here.
The chamber is distributed by PopNeuron LLC, under license from the University of Colorado.
www.popneuron.com
3. Closed Loop Optogenetic Control
With the recent advancement of optogenetic control, control of neural circuits with light has become possible. One application of this method is closed-loop optogenetic control, i.e. light based intervention in response to certain activity patterns in the brain. Our device combines neural recordings with a circuit that can be used to deliver light pulses in response to certain brain states.
Patent:
T.C. Lei, A. Klug, S.H. Pun, C.H. Chen, M.I. Vai, P.U. Mak, and E.A. McCullagh:
An integrated circuit for simultaneous electrophysiology recording and optogenetic neural control.
Awarded 05-21-2021. U.S. Patent Number 11,000,225. PDF here.
4. Small Animal Stereotaxy
Targeting a small and deep brain nucleus such as an auditory brain stem nucleus in-vivo is challenging. Some of these nuclei are 7-10 mm below the surface and are only 0.5 mm in diameter such that an electrode, injection pipette, or other tool that needs to be advanced into these nuclei needs to have an accuracy of better than 1 degree. We developed a novel stereotaxic device for use with small animals such as rodents, which aims to overcome many of the existing challenges associated with stereotaxic work in laboratory settings.
Supported by a NIH small business grant (STTR R41 NS 119079, 2020-2022) awarded to PopNeuron LLC with a subcontract between the business and the university.
More information can be found at:
www.stereotax.com
The technology is distributed by PopNeuron LLC, under license from the University of Colorado.
www.popneuron.com
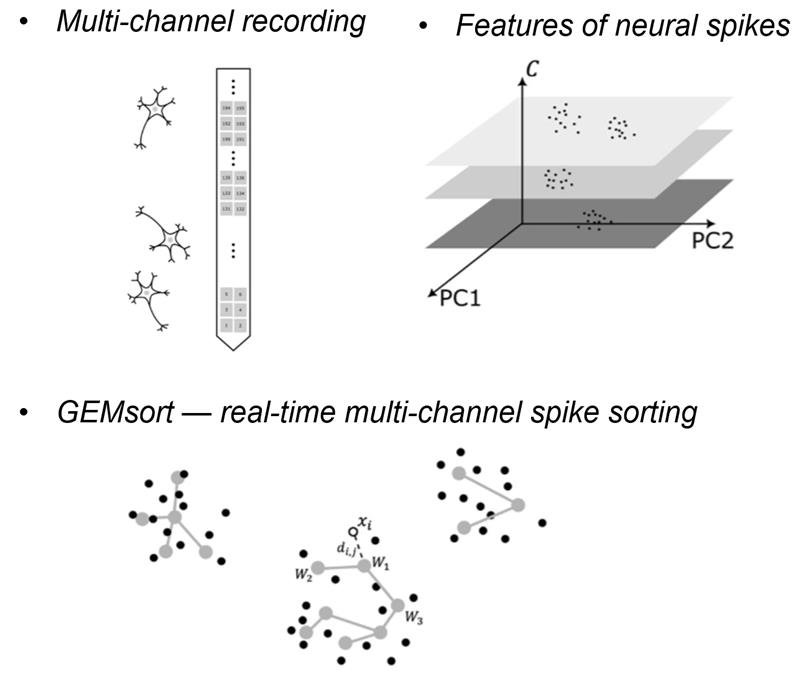
5. Spike sorting
Recording patterns of electrical activity from neurons is one of the most important methods to study brain function. This is typically done with an electrode placed in the brain area of interest. This electrode can pick up neural spikes from several nearby neurons resulting in so-called “multi-unit” activity in the recording trace. Spike sorting algorithms are then used to separate this multi-unit activity into several sets of “single-unit” activities, each of which represents the action potential firing pattern of a single neuron.
While many powerful spike sorting algorithms have existed for many years and have been used by many investigators, one critical gap is becoming more pronounced with the advent of multi- and high-channel-count hardware. Due to the relatively high computational demands of these algorithms, live spike sorting of multichannel data has been challenging, if not impossible. Most existing sorting algorithms are not designed to sort neural spikes in real-time as they are being recorded. Another problem is that sorting algorithms require high-performance computers equipped with significant amounts of computational power and system memory to run, also making them post-hoc and unsuitable for live applications. This challenge has greatly increased with the recent advent of multi-channel and high-channel recording systems. These systems are capable of recording neural spikes from up to several hundred or even thousand channels at the same time. Such datasets are typically examined post-hoc or alternatively, near-live sorting of a very small subset of the incoming data is performed. At the same time, it is critical for many applications to receive single neuron feedback during an experiment both for quality control of the incoming data and to allow the investigator to adapt the experiment based on the incoming data. If we had technology that could perform real-time spike sorting for these vast amounts of incoming data, we could attempt new types of experimental designs that are out of reach with today’s technology.
Our group recently developed a novel, powerful, yet efficient and computationally inexpensive spike sorting algorithm, which -for the first time- allows for live spike sorting of even high and ultra-high channel data streams in their entirety. Termed “EGNG” for single channel sorting and “GEMsort” for multichannel sorting, this algorithm can sort massive amounts of neural spikes live and with negligible processing latency.
For more information, refer to Mohammadi et. al. 2024.
With the support of a NIH Small Business Award (STTR R41 NS 132700) we are bringing this sorting technology to research labs interested in using this algorithm.