Research Projects:
Our laboratory studies the mammalian sound localization pathway, a circuit in the auditory brainstem which is involved in telling us “where” in space a sound is coming from. This circuit is critical for animals and humans to localize sound. Additionally, it plays a key role in helping us spatially separate multiple simultaneous and competing sounds from each other, thereby helping us function in typical “cocktail party” situations. We would like to understand how this circuit operates in the healthy auditory system, and how it changes in conditions such as age-related central hearing loss or autism spectrum disorder (ASD).
1. Mechanisms of low frequency sound localization
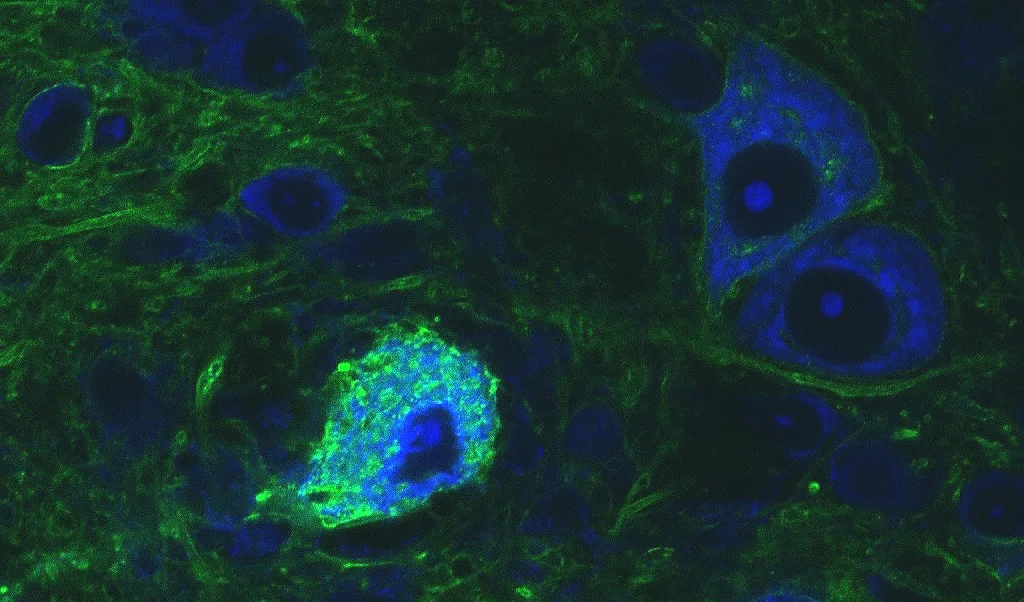
The sound localization pathway consists of two main localization nuclei, the medial superior olive and lateral superior olive (MSO and LSO, respectively). Both receive fast, well-timed inhibitory input from a third nucleus, the medial nucleus of the trapezoid body (MNTB; image 1). We study the functional role of this inhibitory input from MNTB to MSO and LSO in sound localization processing. Our methodology includes but is not limited to in-vivo physiology, in-vitro physiology, behavioral testing, optogenetics, and neuronal modeling.
Image 1
Confocal image of MNTB section from gerbil injected with optogenetic viral constructs to test expression levels and longevity of the expression. Gerbil was injected with AAV 5 eNpHR 3.0 and this image was taken after 75 days.
2. Alterations in the sound localization pathway in aging
The very precise setup of the sound localization pathway changes with age, both in human subjects and animal models. Older listeners very commonly complain about difficulties following a conversation of interest in noisy environments such as busy restaurants. We would like to know exactly what these changes are, how they happen, and how they affect information processing performed by this brain circuit. This information is needed for the development of future solutions for this type of age-related hearing loss. Our methodology includes in-vitro physiology, behavioral testing, immunohistochemistry, microscopy, including CARS microscopy, optogenetics, and human testing.
Image 2
Four images of different MNTB neurons (green) with afferent calyces of Held (orange). The calyx of Held is a type of giant synapse, which synapses onto MNTB neurons and relays excitatory information to these neurons. The size of the synapse, its particular subcellular design, the location of ion channels, and its vesicles and receptors all contribute to the extreme temporal precision of information relayed across this synapse. After receiving the excitatory inputs, the MNTB principal neurons in turn send inhibitory outputs to a number of targets in the auditory brain stem. Thus, the MNTB acts as a master source of well-timed inhibition for the lower auditory system, especially the sound localization pathway. Visualization of the calyx of Held via tracer injection (tetramethylrhodamine dextran) into the cochlear nucleus, MNTB neurons were labeled with fluorescent Nissl label.
3. Reversal of central hearing loss
Most recently, we have been able to restore age-related loss of sound localization abilities in animal models. Will the same treatment also work in human listeners? This will be the main question of a clinical trial in preparation, with an anticipated start sometime in 2025. Interested in participating? Feel free to reach out!
Image 3
An example of a “cocktail party situation”. If the man sitting at the center had normal hearing function, it would be easy for him to have a conversation with the woman sitting next to him. If he had central hearing loss, his brain would not be able to discriminate between auditory stimuli coming from the woman and the bar behind her, or perhaps even the man sitting across the table. This would lead to confusion and a lack of normal communication between these individuals.
4. Alterations in the sound localization pathway in ASD and Fragile X
Similar to central hearing loss in aging, the precise setup of the sound localization pathway is altered in ASD and Fragile X. In fact, the sub cellular alterations are surprisingly similar in the two conditions. At the same time, listeners with ASD also very commonly complain about an inability to follow a conversation of interest in noisy environments. Similar as with age-related central hearing loss, a key requirement for developing future solutions is a thorough understanding of these alterations and their impact on the overall neural circuit function. Our methodology includes in-vitro physiology, behavioral testing, immunohistochemistry, and microscopy, including CARS, and super-resolution microscopy.
Image 4
A CARS microscopy image from an FMR1 knockout mouse brain. When compared to a wild type mouse brain, we observed a decrease in myelination diameter. This decrease in myelination in the sound localization pathway affects temporal processing, resulting in the same auditory stimuli discrimination difficulties seen in central hearing loss.
5. Tool development and engineering
A number of these experiments require specialized tools which are not readily commercially available. For example, the targeting of small, deep brain nuclei such as the sound localization areas in the brain stem is challenging in-vivo. We developed a stereotaxic device with increased navigational precision to increase the targeting accuracy for these brain areas. Also, delivering light to these deep and optically dense brain areas for optogenetic manipulation requires a good understanding of the light scattering properties of these specific areas (brain tissue is highly diverse in terms of optical density). We studied the scattering coefficients of many brain areas and published a light scattering brain atlas with associated APP. Other tools include a slice chamber for ultra-low bath volumes and a novel spike-sorting technology that is capable of live spike sorting data from many channels simultaneously, including high and ultra-high channel systems such as NeuroPixels. Finally, we participated in the development of a novel sapphire-based recording electrode which combines multiple recordings sites with several small LED sites next to each other on the same substrate. The engineering projects are in collaboration with Dr. Tim Lei from the Department of Electrical Engineering at UD Denver.
Image 5
Fully automated robotic stereotaxic system based on 3D structure and geometrical triangulation. Accurately targeting deep brain nuclei (deeper than 7mm from the dorsal surface of the brain) is very difficult with traditional methods and requires a near perfect entry angle. Doing this by hand is unreliable. We solved this issue by creating a 6-legged stereotax paired with an advanced 3D modeling software. This allows us to reliably target the deep brain nuclei that are responsible for sound localization.
Tools and Methods used in the lab:
Our laboratory uses a combination on in-vitro electrophysiology, in-vivo electrophysiology, anatomical and immunohistochemical methods, optogenetics and behavioral testing. In-vitro electrophysiology (patch clamp) is a great tool to study information processing in neurons on a cellular and subcellular level. We use patch clamp recordings to study synapses, ion channels, and the interaction of excitation and inhibition on a subcellular level. Patch clamp data informs neuronal modeling which we use as an additional control to test our understanding of the neural circuits we investigate. By contrast, in-vivo electrophysiology (extracellular recordings) allows us to study, on a systems level, how sound information is processed (image 2, left device). Immunohistochemistry and neural tracing methods allow us to study the connections between different brain areas, and thus understand the ‘wiring’ of the auditory system. Viral manipulations and optogenetics allow us to manipulate neural circuits with light. We express light sensitive ion channels in auditory neurons, and then either turn off these cells with light (when an inhibitory protein, e.g. halorhodopsin, was expressed), or turn on the cells with light (when an excitatory protein, e.g. channelrhodopsin, was expressed). Light is delivered to deep brain nuclei via glass fibers that are connected to lasers or LEDs (image 2, right device). In order to understand, how light that is delivered by these fibers will spread in brain tissue, we also study the light scattering properties of brain tissue (Optogenetics APP, see Tech Transfer). Finally, we test sound localization abilities of both human and animal subjects with behavioral testing.