Research Projects:
Our laboratory studies the mammalian sound localization pathway, a circuit in the auditory brainstem which is involved in telling us “where” in space a sound is coming from. This circuit is important for an animal or human to localize sound, but it is also important to help us spatially separate multiple simultaneous and competing sounds from each other, and thus help us function in typical “cocktail party” situations. We would like to understand how this circuit operates in the healthy auditory system, and how it changes in medical conditions such as central hearing loss or autism spectrum disorder (ASD).
1. Mechanisms of low frequency sound localization
The sound localization pathway consists of two main localization nuclei, the medial superior olive and lateral superior olive (MSO and LSO, respectively). Both receive fast and well-timed inhibitory input from a third nucleus, the medial nucleus of the trapezoid body (image 1). We study the functional role of this inhibitory input from MNTB to MSO and LSO in sound localization processing. The methodology includes in-vivo physiology, in-vitro physiology, behavioral testing, optogenetics, neuronal modeling.
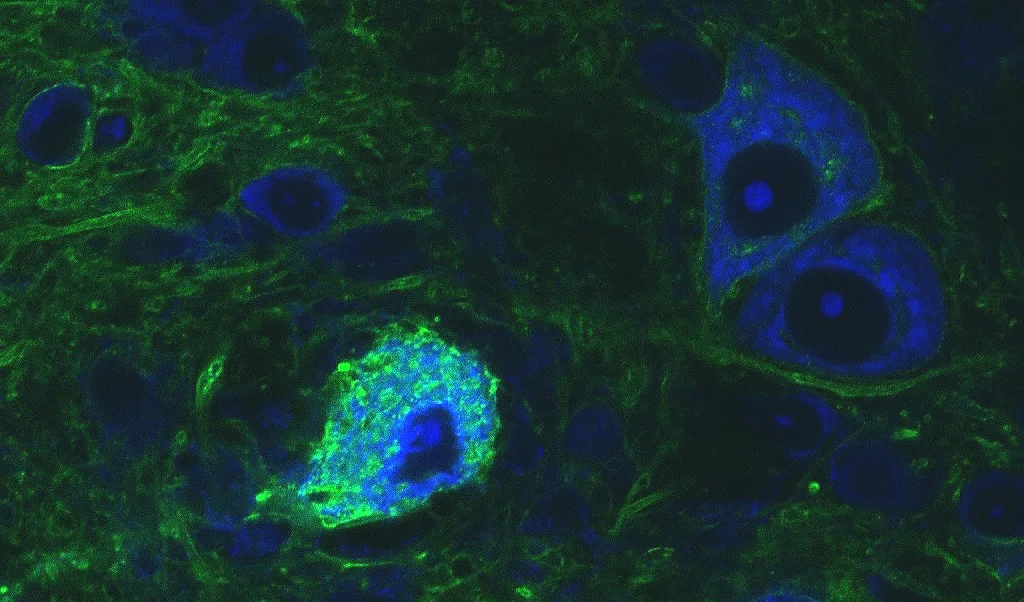
Image 1
Four images of different MNTB neurons (green) with afferent calyces of Held. The calyx of Held (orange) is a type of giant synapse, which synapses onto MNTB neurons and relays excitatory information to these neurons. The size of the synapse, its particular subcellular design, the location of ion channels, vesicles and receptors all contribute to the relaying of information across this synapse with extreme temporal precision. After receiving the excitatory inputs, the MNTB principal neurons in turn send inhibitory outputs to a number of targets in the auditory brain stem and thus act as a master source of well-timed inhibition for the lower auditory system, especially the sound localization pathway. Visualization of the calyx of Held via tracer injection (tetramethylrhodamine dextran) into the cochlear nucleus, MNTB neurons were labeled with fluorescent Nissl label.
2. Alterations in the sound localization pathway in central hearing loss
The very precise setup of the sound localization pathway changes with age, both in human subjects and animal models. We would like to know exactly what these changes are, and how they affect information processing performed by this circuit. Specifically, we are interested in the question how these alterations affect the circuit’s performance in cocktail party environments. This information is needed for the development of future treatments for central hearing loss. The methodology includes in-vitro physiology, behavioral testing, immunohistochemistry, microscopy including CARS microscopy, optogenetics and human testing.
3. Alterations in the sound localization pathway in ASD and Fragile X
Similar as with central hearing loss, the precise setup of the sound localization pathway is altered in ASD and Fragile X, resulting in similar difficulties in affected patients and animal models to localize sound and perform in cocktail party situations. Similar as with central hearing loss, a requirement for the development of future treatments is a good understanding of these alterations and how they affect the performance of the overall neural circuit. The methodology includes in-vitro physiology, behavioral testing, immunohistochemistry, and microscopy including CARS and super-resolution microscopy.
4. Tool development and engineering
A number of these experiments require specialized tools which are not readily commercially available. For example, the targeting of small and deep brain nuclei such as the sound localization areas in the brain stem is challenging in-vivo. We are developing a stereotaxic device with increased navigational precision to increase the targeting accuracy for these brain areas. Also, delivering light to these deep and optically dense brain areas for optogenetic manipulation requires a good understanding of the light scattering properties of these specific areas (brain tissue is highly diverse in terms of optical density). We studied the scattering coefficients of many brain areas and published a light scattering brain atlas with associated APP. The engineering projects are in collaboration with Dr. Tim Lei from the Department of Electrical Engineering at UD Denver.
Image 2
Image of a multibarrel electrode (left) and a glass fiber implant (right). A match is also shown on the image for size comparison. The multi barrel electrode consists of s single barrel recording electrode that can be used to record electrical activity from single neurons in-vivo. The attached multibarrel (in this case a 5 barrel unit) can be filled with various pharmacological agents (in most cases agonists and antagonists of excitatory or inhibitory receptors). The drugs can be iontophoresed on demand into the immediate vicinity of the neuron, thus activating or deactivating certain inputs that the neuron is receiving. The glass fiber tip can be implanted into the brain nucleus of interest and used to deliver light to this nucleus, also to manipulate the neural circuitry under investigation.
Tools and Methods used in the lab:
Our laboratory uses a combination on in-vitro electrophysiology, in-vivo electrophysiology, anatomical and immunohistochemical methods, optogenetics and behavioral testing. In-vitro electrophysiology (patch clamp) is a great tool to study information processing in neurons on a cellular and subcellular level. We use patch clamp recordings to study synapses, ion channels, and the interaction of excitation and inhibition on a subcellular level. Patch clamp data informs neuronal modeling which we use as an additional control to test our understanding of the neural circuits we investigate. By contrast, in-vivo electrophysiology (extracellular recordings) allows us to study on a systems level, how sound information is processed (image 2, left device). Immunohistochemistry and neural tracing methods allow us to study the connections between different brain areas, and thus understand the ‘wiring’ of the auditory system. Viral manipulations and optogenetics allow us to manipulate neural circuits with light. We express light sensitive ion channels in auditory neurons, and then either turn off these cells with light (when an inhibitory protein, e.g. halorhodopsin, was expressed), or turn on the cells with light (when an excitatory protein, e.g. channelrhodopsin, was expressed). Light is delivered to deep brain nuclei via glass fibers that are connected to lasers or LEDs (image 2, right device). In order to understand, how light that is delivered by these fibers will spread in brain tissue, we also study the light scattering properties of brain tissue (Optogenetics APP, see Tech Transfer). Finally, we test sound localization abilities of both human and animal subjects with behavioral testing.